
Samsung just announced its latest smartwatch, the Galaxy Watch 3, at its second Galaxy Unpacked event of the year. Once again, Samsung’s made the best smartwatch for Android users, and it doesn’t even run Google’s Wear OS. The Samsung Galaxy Watch 3 packs loads of fitness features, but three of its best health monitoring features won’t be available in every country at launch. That’s because they require approval from the regulatory agencies of every country, and in a surprise announcement on stage, Samsung says that they just got clearance from the U.S. FDA for the Galaxy Watch 3 and Watch Active 2’s ECG monitoring app.
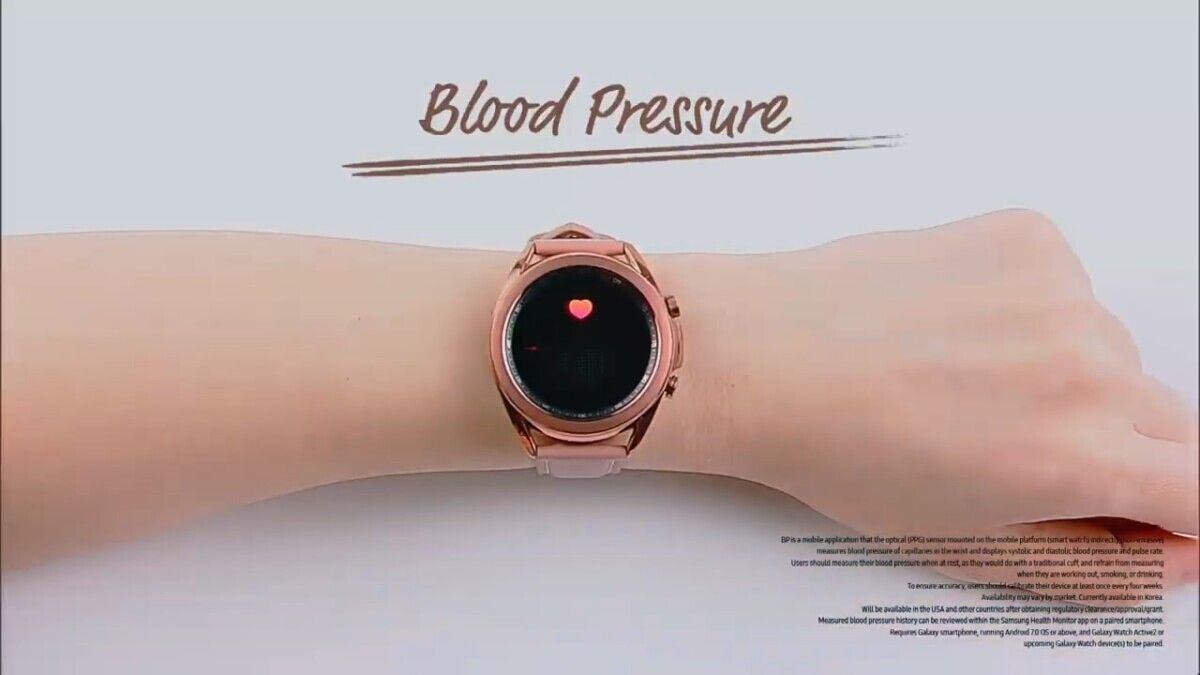
“And I’m excited to announce that we have just received the U.S. FDA clearance for Samsung ECG monitor app,” said Federico Casalegno, SVP of Experience Planning and SDIC. ECG monitoring will allow users to monitor their heart rhythm for irregularities such as atrial fibrillation (Afib), which is one of the leading causes of strokes. Meanwhile, blood pressure monitoring allows users to check for irregularities such as high blood pressure (hypertension) or low blood pressure (hypotension), which are precursors to many other diseases.
Samsung previously announced that they had received clearance from South Korea’s MFDS for blood pressure and ECG monitoring in the country, but the company today repeatedly showed disclaimers that these two features will not be available in the U.S. until they received clearance from the FDA. Apparently, Samsung just managed to receive that clearance, so we can expect to see blood pressure and ECG monitoring roll out soon for the Galaxy Watch Active 2 and Galaxy Watch 3.


Lastly, one of the other health monitoring features of the new Galaxy Watch 3, blood oxygen monitoring, will not be available in every country. Samsung says that the feature is not supported in Japan, Angola, South Africa, Thailand, Iran, Libya, France, Algeria, or Canada. Sp02 monitoring should be available in all other countries where the Galaxy Watch 3 is sold, though.
| Specifications | Samsung Galaxy Watch 3 |
|---|---|
| Dimensions & Weight |
|
| Display |
|
| Watchband size |
|
| Memory | 1GB RAM, 8GB internal storage |
| Connectivity |
|
| Other features |
|
| Sensors |
|
| NFC Payments | Yes, Samsung Pay |
| Battery |
|
| Durability | 5ATM + IP68/ MIL-STD-810G |
| OS | Tizen Based Wearable OS 5.5 |
| Colors |
|
The post Samsung says they just got U.S. FDA approval for the Galaxy Watch 3’s ECG monitoring app appeared first on xda-developers.


0 comments:
Post a Comment